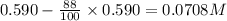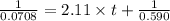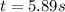Consider this reaction: →2NH3g+N2g3H2g At a certain temperature it obeys this rate law: rate =2.11·M−1s−1NH32 Suppose a vessel contains NH3 at a concentration of 0.590M. Calculate how long it takes for the concentration of NH3 to decrease by 88.0%. You may assume no other reaction is important.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Consider this reaction: →2NH3g+N2g3H2g At a certain temperature it obeys this rate law: rate =2.11·M...
Questions


Mathematics, 26.04.2021 19:30

Mathematics, 26.04.2021 19:30

Biology, 26.04.2021 19:30





Spanish, 26.04.2021 19:30

Mathematics, 26.04.2021 19:30

Mathematics, 26.04.2021 19:30


Mathematics, 26.04.2021 19:30

Mathematics, 26.04.2021 19:30

Mathematics, 26.04.2021 19:30

Computers and Technology, 26.04.2021 19:30

English, 26.04.2021 19:30


History, 26.04.2021 19:30

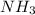 to decrease by 88.0%.
to decrease by 88.0%. 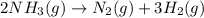
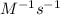 , the kinetics must be second order.
, the kinetics must be second order.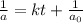
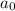 = initiaal concentration = 0.590 M
= initiaal concentration = 0.590 M