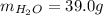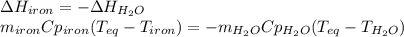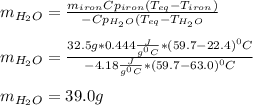Chemistry, 20.03.2020 01:28 ghari112345
A 32.5 g iron rod, initially at 22.4 ∘C, is submerged into an unknown mass of water at 63.0 ∘C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 59.7 ∘C. Part A What is the mass of the water?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1


Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

You know the right answer?
A 32.5 g iron rod, initially at 22.4 ∘C, is submerged into an unknown mass of water at 63.0 ∘C, in a...
Questions

Biology, 03.03.2021 03:40


Mathematics, 03.03.2021 03:40


Mathematics, 03.03.2021 03:40






Mathematics, 03.03.2021 03:40


Mathematics, 03.03.2021 03:40


History, 03.03.2021 03:40

Mathematics, 03.03.2021 03:40


History, 03.03.2021 03:40