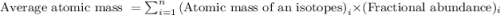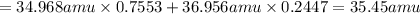Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3

Chemistry, 23.06.2019 09:00
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2
You know the right answer?
The atomic masses of 35-Cl (75.53 percent) and 37-Cl (24.47 percent) are 34.968 amu and 36.956 amu,...
Questions

Law, 15.03.2020 10:18

Mathematics, 15.03.2020 10:25

Mathematics, 15.03.2020 10:25

Mathematics, 15.03.2020 10:26

Chemistry, 15.03.2020 10:27



English, 15.03.2020 10:28




Physics, 15.03.2020 10:41


Law, 15.03.2020 10:43


Mathematics, 15.03.2020 10:45

Mathematics, 15.03.2020 10:49

Social Studies, 15.03.2020 10:49

Mathematics, 15.03.2020 10:50