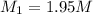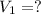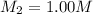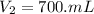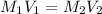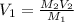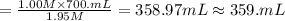A chemist must prepare 700.mL of 1.00M aqueous calcium bromide CaBr2 working solution. He'll do this by pouring out some 1.95M aqueous calcium bromide stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the calcium bromide stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
A chemist must prepare 700.mL of 1.00M aqueous calcium bromide CaBr2 working solution. He'll do this...
Questions



Mathematics, 17.04.2020 23:01


Mathematics, 17.04.2020 23:01

Geography, 17.04.2020 23:02







Mathematics, 17.04.2020 23:02

History, 17.04.2020 23:02

Mathematics, 17.04.2020 23:02

English, 17.04.2020 23:02




Biology, 17.04.2020 23:02