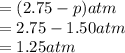At 250°C the equilibrium constant (Kp) for the reaction below is 1.81. PCl5(g) PCl3(g) + Cl2(g) Sufficient PCl5 is put into a vessel to give an initial pressure of 2.75 atm at 250°C. What will be the final pressure at this temperature after the system has reached equilibrium?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
At 250°C the equilibrium constant (Kp) for the reaction below is 1.81. PCl5(g) PCl3(g) + Cl2(g) Suff...
Questions







Mathematics, 09.09.2020 20:01

Mathematics, 09.09.2020 20:01


Mathematics, 09.09.2020 20:01


Mathematics, 09.09.2020 20:01

Mathematics, 09.09.2020 20:01

Mathematics, 09.09.2020 20:01

Mathematics, 09.09.2020 20:01

English, 09.09.2020 20:01



History, 09.09.2020 20:01

 at equilibrium is 1.25 atm
at equilibrium is 1.25 atm


 for the given reaction
for the given reaction