
Chemistry, 20.03.2020 02:56 elijahdouglass00
How many moles of solid NaF would have to be added to 1.0 L of 1.78 M HF solution to achieve a buffer of pH 3.35? Assume there is no volume change. (Ka for HF = 7.2 10-4)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3


Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
How many moles of solid NaF would have to be added to 1.0 L of 1.78 M HF solution to achieve a buffe...
Questions

Mathematics, 09.04.2020 18:53

Mathematics, 09.04.2020 18:53

Mathematics, 09.04.2020 18:53


World Languages, 09.04.2020 18:53

Mathematics, 09.04.2020 18:53

Mathematics, 09.04.2020 18:53

Mathematics, 09.04.2020 18:53



Mathematics, 09.04.2020 18:53



Mathematics, 09.04.2020 18:53


Mathematics, 09.04.2020 18:53




Advanced Placement (AP), 09.04.2020 18:54

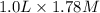
![pK_{a} + log (\frac{[NaF]}{[HF]})](/tpl/images/0555/2664/2271c.png)
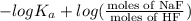
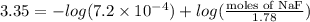
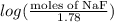 = 0.208
= 0.208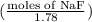 = 1.614
= 1.614

