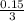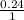Chemistry, 20.03.2020 02:58 vegeta8375
Two solutions are created by mixing one solution containing lithium nitrate with one containing sodium phosphate.
Solution A is created by mixing 0.500 L of 0.30 M LiNO3 with 0.400 L of 0.20 M Na3PO4.
Solution B is created by mixing 0.400 L of 0.20 M LiNO3 with 0.500 L of 0.30 M Na3PO4.
In which of these solutions will lithium phosphate precipitate? Lithium phosphate has a Ksp = 2.3x10−4 Select the solution(s) that will form a precipitate.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Two solutions are created by mixing one solution containing lithium nitrate with one containing sodi...
Questions



Biology, 22.07.2019 13:20

Business, 22.07.2019 13:20




History, 22.07.2019 13:20

English, 22.07.2019 13:20

Health, 22.07.2019 13:20



Social Studies, 22.07.2019 13:20

English, 22.07.2019 13:20


Physics, 22.07.2019 13:20


Business, 22.07.2019 13:20