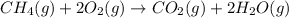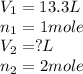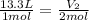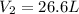Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1


You know the right answer?
How many liters of water vapor can be produced if 13.3 liters of methane gas (ch4) are combusted, if...
Questions



Spanish, 22.11.2019 19:31

Mathematics, 22.11.2019 19:31


Mathematics, 22.11.2019 19:31

History, 22.11.2019 19:31

English, 22.11.2019 19:31


Mathematics, 22.11.2019 19:31








Social Studies, 22.11.2019 19:31

Mathematics, 22.11.2019 19:31

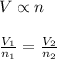
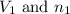 = Initial volume and number of moles
= Initial volume and number of moles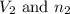 = Final volume and number of moles
= Final volume and number of moles