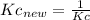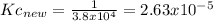Chemistry, 20.03.2020 05:08 zairaefh3200
Knowing that the equilibrium constant for the reaction below H2 (g) + Br2 (g) ⇌ 2 HBr (g) is Kc = 3.8 × 104, determine the equilibrium constant for the following reaction: 4 HBr (g) ⇌ 2 H2 (g) + 2 Br2 (g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
Knowing that the equilibrium constant for the reaction below H2 (g) + Br2 (g) ⇌ 2 HBr (g) is Kc = 3....
Questions

English, 25.07.2019 03:30

Mathematics, 25.07.2019 03:30



History, 25.07.2019 03:30

Physics, 25.07.2019 03:30






Physics, 25.07.2019 03:30


English, 25.07.2019 03:30


English, 25.07.2019 03:30


History, 25.07.2019 03:30


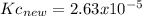
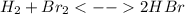
![Kc = \frac{[Products]^{x} }{[Reagents]^{y} } \\](/tpl/images/0555/4796/ac561.png)
![Kc = \frac{[HBr]^{2} }{[H_{2}] * [Br_{2}] }](/tpl/images/0555/4796/e8a20.png) and the value of Kc is Kc =
and the value of Kc is Kc = 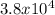
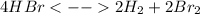
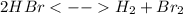 ,
, 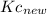 is:
is:![Kc_{new} = \frac{H_{2}*Br_{2} }{[HBr]^{2} }](/tpl/images/0555/4796/5e6f8.png)