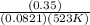A sample of gaseous PCl5 was introduced into an evacuated flask so that the pressure of pure PCl5 would be 0.50 atm at 523 K. However, PCl5 decomposes to gaseous PCl3 and Cl2, and the actual pressure in the flask was found to be 0.78 atm. Calculate Kp for the decomposition reaction PCl5(g) PCl3(g) Cl2(g) at 523 K. Also, calculate K at this temperature.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2


Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
A sample of gaseous PCl5 was introduced into an evacuated flask so that the pressure of pure PCl5 wo...
Questions



Biology, 16.07.2019 09:30







History, 16.07.2019 09:30


Mathematics, 16.07.2019 09:30








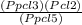 Kp =
Kp = 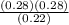 Kp = 0.35
Kp = 0.35