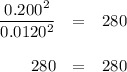Chemistry, 20.03.2020 06:29 lisafrench8222
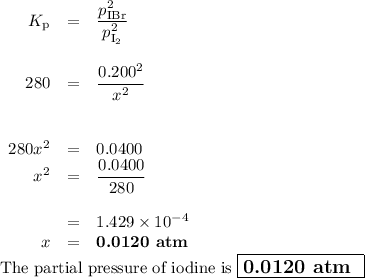
The equilibrium constant Kp for the reaction I2(g) + Br2(g) ⇀↽ 2 IBr(g) + 11.7 kJ is 280 at 150◦C. Suppose that a quantity of IBr is placed in a closed reaction vessel and the system is allowed to come to equilibrium at 150◦C. When equilibrium is established, the pressure of IBr is 0.200 atm. What is the pressure of I2 at equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 23.06.2019 15:30
Express your answer using two significant figures. 1.7 km^2
Answers: 2

Chemistry, 23.06.2019 21:50
41. we want to mark off a thermometer in both celsius and fahrenheit temperatures. on the celsius scale, the lowest temperature mark is at and the highest temperature mark is at 50 °c. what are the equivalent fahrenheit temperatures? petrucci, ralph h.. general chemistry (p. 28). pearson education. kindle edition.
Answers: 3
You know the right answer?
The equilibrium constant Kp for the reaction I2(g) + Br2(g) ⇀↽ 2 IBr(g) + 11.7 kJ is 280 at 150◦C. S...
Questions



Mathematics, 18.07.2019 17:50

Mathematics, 18.07.2019 17:50

Chemistry, 18.07.2019 17:50

Social Studies, 18.07.2019 17:50



Mathematics, 18.07.2019 17:50

Mathematics, 18.07.2019 17:50


Mathematics, 18.07.2019 17:50

Mathematics, 18.07.2019 17:50

Mathematics, 18.07.2019 17:50


Mathematics, 18.07.2019 17:50

Biology, 18.07.2019 17:50

Mathematics, 18.07.2019 17:50

Chemistry, 18.07.2019 17:50

History, 18.07.2019 17:50

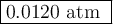
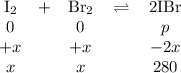
 }
}