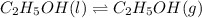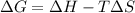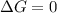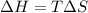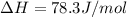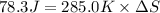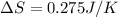Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Calculate the standard entropy of vaporization of ethanol, C2H5OH, at 285.0 K, given that the molar...
Questions

History, 21.04.2021 23:40


Mathematics, 21.04.2021 23:40

Mathematics, 21.04.2021 23:40





Mathematics, 21.04.2021 23:40

Social Studies, 21.04.2021 23:40



Mathematics, 21.04.2021 23:40


Health, 21.04.2021 23:50

Biology, 21.04.2021 23:50

Mathematics, 21.04.2021 23:50

Mathematics, 21.04.2021 23:50


History, 21.04.2021 23:50