
Chemistry, 20.03.2020 08:30 darwin59651
Calculate the pH of a 0.437 M aqueous solution of hydrocyanic acid (HCN, Ka = 4.0×10-10) and the equilibrium concentrations of the weak acid and its conjugate base.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1
You know the right answer?
Calculate the pH of a 0.437 M aqueous solution of hydrocyanic acid (HCN, Ka = 4.0×10-10) and the equ...
Questions


Biology, 13.12.2021 06:20

History, 13.12.2021 06:20


History, 13.12.2021 06:20


Mathematics, 13.12.2021 06:20

Advanced Placement (AP), 13.12.2021 06:20

Health, 13.12.2021 06:20


English, 13.12.2021 06:20


Mathematics, 13.12.2021 06:20




History, 13.12.2021 06:20


Mathematics, 13.12.2021 06:20

![[HCN]_{eq}=0.43699M](/tpl/images/0555/6850/8a21f.png)
![[CN^-]_{eq}=1.322x10^{-5}M](/tpl/images/0555/6850/83c77.png)
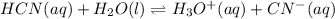
![Ka=\frac{[H^+]_{eq}[CN^-]_{eq}}{[HCN]_{eq}}](/tpl/images/0555/6850/4425b.png)
 due to the reaction extent, goes:
due to the reaction extent, goes: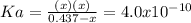
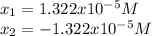
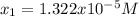
![pH=-log([H^+])=-log(1.322x10^{-5})=4.88](/tpl/images/0555/6850/f810b.png)
![[HCN]_{eq}=0.437M-1.322x10^{-5}M=0.43699M](/tpl/images/0555/6850/d4f3f.png)


