
Chemistry, 20.03.2020 09:57 nommies005
4. A solution of the weak base, ammonia (NH3), was completely neutralized with the strong acid HCl. Write out the dominant equilibrium (including phase labels) that would exist in this "neutralized" solution. HINT: first look at what ions would exist in solution after the non-equilibrium reaction with the strong acid is over. Second, decide if either of these ions is an acid or a base. Lastly, write the equation for this ion acting as an acid or base in water.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
4. A solution of the weak base, ammonia (NH3), was completely neutralized with the strong acid HCl....
Questions


Mathematics, 30.05.2020 01:58

Mathematics, 30.05.2020 01:58

Mathematics, 30.05.2020 01:58

Mathematics, 30.05.2020 01:58



Chemistry, 30.05.2020 01:58

Mathematics, 30.05.2020 01:58



Mathematics, 30.05.2020 01:58

English, 30.05.2020 01:58

Chemistry, 30.05.2020 01:58

Mathematics, 30.05.2020 01:58


Mathematics, 30.05.2020 01:58

Mathematics, 30.05.2020 01:58


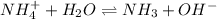
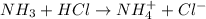
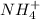 and
and 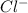 ions exist.
ions exist.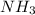 is an weak base and HCl is a strong acid.
is an weak base and HCl is a strong acid.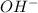 .
.

