
Chemistry, 20.03.2020 10:19 emilypk1998
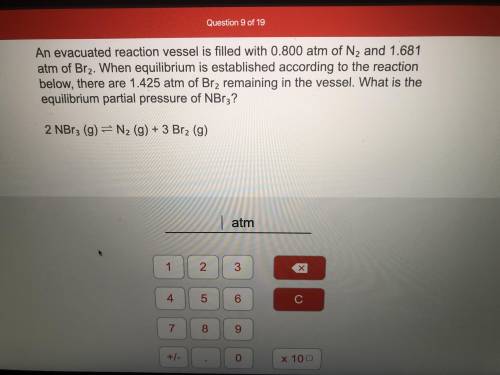
An evacuated reaction vessel is filled with 0.800atm of N2 and 1.681atm of Br2. When equilibrium is established according to the reaction below, there are 1.425atm of Br2 remaining in the vessel. What is the equilibrium partial pressure of NBr3?
2 NBr3(g) <————> N2(g) + 3 Br2(g)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
An evacuated reaction vessel is filled with 0.800atm of N2 and 1.681atm of Br2. When equilibrium is...
Questions

History, 21.04.2020 00:05

Mathematics, 21.04.2020 00:05




History, 21.04.2020 00:05

English, 21.04.2020 00:05


History, 21.04.2020 00:05

Chemistry, 21.04.2020 00:05

Mathematics, 21.04.2020 00:05


Mathematics, 21.04.2020 00:05



Health, 21.04.2020 00:06

English, 21.04.2020 00:06

Health, 21.04.2020 00:06

Mathematics, 21.04.2020 00:06



