
Chemistry, 20.03.2020 09:52 KillerSteamcar
In the presence of excess oxygen, methane gas burns in a constant-pressure system Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. to yield carbon dioxide and water: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ΔH = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2

Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
You know the right answer?
In the presence of excess oxygen, methane gas burns in a constant-pressure system Calculate the valu...
Questions

Mathematics, 11.10.2020 08:01

Mathematics, 11.10.2020 08:01

Mathematics, 11.10.2020 08:01

Geography, 11.10.2020 08:01

Mathematics, 11.10.2020 08:01

Physics, 11.10.2020 08:01


History, 11.10.2020 08:01

Mathematics, 11.10.2020 08:01





Chemistry, 11.10.2020 08:01

Mathematics, 11.10.2020 08:01

English, 11.10.2020 08:01

Mathematics, 11.10.2020 08:01

Mathematics, 11.10.2020 08:01


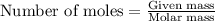
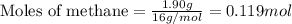


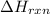 = enthalpy change of the reaction = -890.0 kJ/mol
= enthalpy change of the reaction = -890.0 kJ/mol


