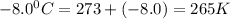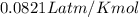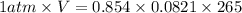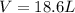Chemistry, 20.03.2020 10:04 brandon56238
A reaction at −8.0°C evolves 854.mmol of boron trifluoride gas. Calculate the volume of boron trifluoride gas that is collected. You can assume the pressure in the room is exactly 1atm. Be sure your answer has the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3
You know the right answer?
A reaction at −8.0°C evolves 854.mmol of boron trifluoride gas. Calculate the volume of boron triflu...
Questions





Mathematics, 29.09.2021 19:40

Mathematics, 29.09.2021 19:40


Mathematics, 29.09.2021 19:40



Biology, 29.09.2021 19:40


English, 29.09.2021 19:40




English, 29.09.2021 19:40

Mathematics, 29.09.2021 19:40