Chemistry, 20.03.2020 10:04 morkitus13
An ion Mn+ has a single electron. The highest energy line in its emission spectrum occurs at a frequency of 8.225 × 1016 Hz. Identify the ion. (Enter the symbol of the element in the first box, and its charge in the second.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2


Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
An ion Mn+ has a single electron. The highest energy line in its emission spectrum occurs at a frequ...
Questions


Mathematics, 06.04.2021 14:00

World Languages, 06.04.2021 14:00



Mathematics, 06.04.2021 14:00

Business, 06.04.2021 14:00



Mathematics, 06.04.2021 14:00

English, 06.04.2021 14:00

Mathematics, 06.04.2021 14:00

Chemistry, 06.04.2021 14:00

Engineering, 06.04.2021 14:00



English, 06.04.2021 14:00


English, 06.04.2021 14:00

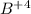
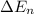 = change in energy
= change in energy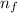 = Higher energy level =
= Higher energy level = 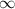
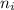 = Lower energy level = 1
= Lower energy level = 1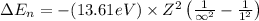
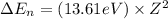 ............(1)
............(1)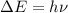
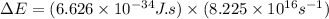
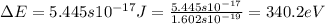 .......(2)
.......(2)