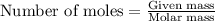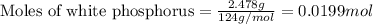Chemistry, 20.03.2020 10:03 xXCoryxKenshinXx
2.478 g of white phosphorus was used to make phosphine according to the equation: P₄(s) + 3OH⁻(aq) + 3H₂O(l) → PH₃(g) + 3H₂PO₂⁻(aq) Calculate the amount, in mol, of white phosphorus

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
2.478 g of white phosphorus was used to make phosphine according to the equation: P₄(s) + 3OH⁻(aq) +...
Questions

Mathematics, 21.04.2021 19:00



Social Studies, 21.04.2021 19:00

Biology, 21.04.2021 19:00



Mathematics, 21.04.2021 19:00

English, 21.04.2021 19:00


History, 21.04.2021 19:00

Health, 21.04.2021 19:00



Mathematics, 21.04.2021 19:00




Mathematics, 21.04.2021 19:00