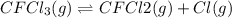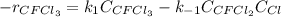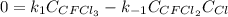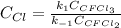Chemistry, 20.03.2020 10:54 diwashkandel6pe02af
Consider the following elementary reaction: CFC13(g)-CFC12(9)+Clg) Suppose we let k1 stand for the rate constant of this reaction, and k1 stand for the rate constant of the reverse reaction Write an expression that gives the equilibrium concentration of Cl in terms of k, k_1, and the equilibrium concentrations of CFCI3 and CFCI2 1. K-1 [ci]

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
Consider the following elementary reaction: CFC13(g)-CFC12(9)+Clg) Suppose we let k1 stand for the r...
Questions








Mathematics, 24.11.2020 20:50

History, 24.11.2020 20:50


English, 24.11.2020 20:50




English, 24.11.2020 20:50

Mathematics, 24.11.2020 20:50

Health, 24.11.2020 20:50


Mathematics, 24.11.2020 20:50

Mathematics, 24.11.2020 20:50

![[Cl]_{eq}=\frac{k_1[CFCl_3]_{eq}}{k_{-1}[CFCl_2]_{eq}}](/tpl/images/0555/9861/640b9.png)