
Chemistry, 20.03.2020 10:26 cearadenney7067
The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy Ea= 50.0 KJ/mol. If the rate constant of this reaction is 0.14M-1 s-1 at -8.0what will the rate constant be at 56.0 ?
Round your answer to significant digits
k= M-1s-1

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an acti...
Questions



Mathematics, 25.02.2020 17:29

Mathematics, 25.02.2020 17:29









English, 25.02.2020 17:30

History, 25.02.2020 17:30


Mathematics, 25.02.2020 17:30




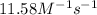
![\ln(\frac{K_{56^oC}}{K_{-8^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0555/9521/c3b12.png)
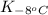 = equilibrium constant at -8°C =
= equilibrium constant at -8°C = 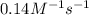
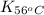 = equilibrium constant at 56°C = ?
= equilibrium constant at 56°C = ?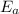 = Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)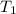 = initial temperature =
= initial temperature = ![-8^oC=[273-8]K=265K](/tpl/images/0555/9521/87944.png)
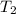 = final temperature =
= final temperature = ![56^oC=[273+56]K=329K](/tpl/images/0555/9521/333d6.png)
![\ln(\frac{K_{56^oC}}{0.14})=\frac{50000J}{8.314J/mol.K}[\frac{1}{265}-\frac{1}{329}]\\\\K_{56^oC}=11.58M^{-1}s^{-1}](/tpl/images/0555/9521/de71d.png)


