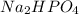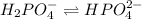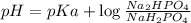Chemistry, 20.03.2020 11:19 MarishaTucker
Buffer consists of 0.50 M NaH2PO4 and 0.40 M Na2HPO4. Phosphoric acid is a triprotic acid (K a 1 = 7.2 × 10 − 3, K a 2 = 6.3 × 10 − 8, and K a 3 = 4.2 × 10 − 13). (a) Which Ka value is most important to this buffer? (b) What is the buffer pH?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3



Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Buffer consists of 0.50 M NaH2PO4 and 0.40 M Na2HPO4. Phosphoric acid is a triprotic acid (K a 1 = 7...
Questions




Biology, 30.11.2020 22:10


Mathematics, 30.11.2020 22:10

English, 30.11.2020 22:10


Mathematics, 30.11.2020 22:10

Mathematics, 30.11.2020 22:10

Mathematics, 30.11.2020 22:10


English, 30.11.2020 22:10


French, 30.11.2020 22:10

Mathematics, 30.11.2020 22:10




Mathematics, 30.11.2020 22:10

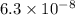 pKa2 = 7.2
pKa2 = 7.2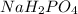 and
and