
Chemistry, 20.03.2020 11:14 ciarrap552
If you assume this reaction is driven to completion because of the large excess of one ion, what is the concentration of [Fe(SCN)]2+ that would be formed from 6.00 mL of 0.00180 M KSCN 5.00 mL 0.240 M Fe(NO3)3 and 14.00 mL of 0.050 M HNO3.
Question 3 options:
0.240 M
4.32 x 10^-4
0.0480
0.0460 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
If you assume this reaction is driven to completion because of the large excess of one ion, what is...
Questions



Mathematics, 26.08.2021 01:00


Biology, 26.08.2021 01:00


Biology, 26.08.2021 01:00

Mathematics, 26.08.2021 01:00

English, 26.08.2021 01:00

Social Studies, 26.08.2021 01:00



Mathematics, 26.08.2021 01:00

Mathematics, 26.08.2021 01:00


Biology, 26.08.2021 01:00




Geography, 26.08.2021 01:00

![[Fe(SCN)]^{2+}](/tpl/images/0556/0307/0c409.png) is,
is, 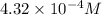
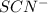 and
and 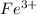 is excess reagent.
is excess reagent.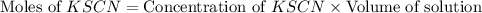
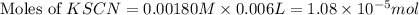
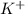 = Moles of
= Moles of 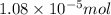
![\text{Concentration of }[Fe(SCN)]^{2+}=\frac{\text{Moles of }[Fe(SCN)]^{2+}}{\text{Volume of solution}}](/tpl/images/0556/0307/e16b4.png)
![\text{Concentration of }[Fe(SCN)]^{2+}=\frac{1.08\times 10^{-5}mol}{0.025L}=4.32\times 10^{-4}M](/tpl/images/0556/0307/cd669.png)


