
Chemistry, 20.03.2020 11:30 vanessa23272
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm? (c = 3.00 × 108 m/s; h = 6.63 × 10–34 J • s; NA = 6.022 × 1023 moles–1)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm...
Questions

Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01


Mathematics, 12.08.2020 05:01




Mathematics, 12.08.2020 05:01



Mathematics, 12.08.2020 05:01





Mathematics, 12.08.2020 05:01





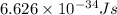
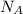 = Avogadro's number =
= Avogadro's number = 
 = wavelength of photon = 486 nm =
= wavelength of photon = 486 nm =  (Conversion factor:
(Conversion factor:  )
)


