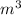Chemistry, 20.03.2020 11:33 BaileyElizabethRay
A 6.30 g 6.30 g sample of solid CaSO 4 ⋅ 2 H 2 O CaSO4⋅2H2O was heated such that the water turned to steam and was driven off. Assuming ideal behavior, what volume would that steam occupy at 1.00 atm 1.00 atm and 100.0 °C?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
A 6.30 g 6.30 g sample of solid CaSO 4 ⋅ 2 H 2 O CaSO4⋅2H2O was heated such that the water turned to...
Questions


Mathematics, 26.01.2021 14:00

Biology, 26.01.2021 14:00


Spanish, 26.01.2021 14:00

English, 26.01.2021 14:00

Mathematics, 26.01.2021 14:00

English, 26.01.2021 14:00


Chemistry, 26.01.2021 14:00

Mathematics, 26.01.2021 14:00

Biology, 26.01.2021 14:00



English, 26.01.2021 14:00

Mathematics, 26.01.2021 14:00

Mathematics, 26.01.2021 14:00

History, 26.01.2021 14:00

Mathematics, 26.01.2021 14:00