
Chemistry, 20.03.2020 11:40 ineedtopeebeforethec
A 8.70 L 8.70 L container holds a mixture of two gases at 33 ° C. 33 °C. The partial pressures of gas A and gas B, respectively, are 0.314 atm 0.314 atm and 0.755 atm. 0.755 atm. If 0.200 mol 0.200 mol of a third gas is added with no change in volume or temperature, what will the total pressure become?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

You know the right answer?
A 8.70 L 8.70 L container holds a mixture of two gases at 33 ° C. 33 °C. The partial pressures of ga...
Questions

Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

Computers and Technology, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00


Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00



Mathematics, 16.11.2020 14:00

History, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00



Geography, 16.11.2020 14:00

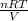 =
= 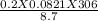 = 0.577 atm
= 0.577 atm


