
Chemistry, 20.03.2020 12:03 cedricevans41p4j3kx
A 140.0-g sample of water at 25.0°C is mixed with 108.6 g of a certain metal at 100.0°C. After thermal equilibrium is established, the (final) temperature of the mixture is 29.6°C. What is the specific heat capacity of the metal, assuming it is constant over the temperature range concerned?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

You know the right answer?
A 140.0-g sample of water at 25.0°C is mixed with 108.6 g of a certain metal at 100.0°C. After therm...
Questions

Health, 05.11.2020 21:40


English, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40




History, 05.11.2020 21:40

Social Studies, 05.11.2020 21:40

History, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40





Mathematics, 05.11.2020 21:40

Social Studies, 05.11.2020 21:40

Law, 05.11.2020 21:40

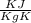
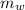 = 140 gm = 0.14 kg
= 140 gm = 0.14 kg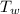 = 25°c = 298 K
= 25°c = 298 K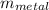 = 108.6 gm = 0.1086 kg
= 108.6 gm = 0.1086 kg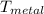 = 100°c = 373 K
= 100°c = 373 K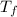 = 29.6°c = 302.6 K
= 29.6°c = 302.6 K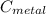 × (
× ( 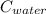 × (
× ( 

