
Chemistry, 20.03.2020 12:25 cupcake20019peehui
2. Hydrogen fluoride can be produced from elemental fluorine and hydrogen according to the reaction H2(g) + F2(g) 2HF(g). The reaction has an equilibrium constant, Kc, of 7.75 x 10 2 at a certain temperature. a. Calculate the equilibrium concentration of HF(g) if 5.750 mol of H2 and F2 are introduced into a 1.500 L flask.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
2. Hydrogen fluoride can be produced from elemental fluorine and hydrogen according to the reaction...
Questions

Biology, 06.10.2019 02:30

Biology, 06.10.2019 02:30


Mathematics, 06.10.2019 02:30

Mathematics, 06.10.2019 02:30



Mathematics, 06.10.2019 02:30

Chemistry, 06.10.2019 02:30



Social Studies, 06.10.2019 02:30


Computers and Technology, 06.10.2019 02:30

Mathematics, 06.10.2019 02:30

Mathematics, 06.10.2019 02:30


Mathematics, 06.10.2019 02:30

Mathematics, 06.10.2019 02:30

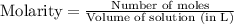
![[H_2]=\frac{5.750 mol}{1.500 L}=3.833 M](/tpl/images/0556/1625/21097.png)
![[F_2]=\frac{5.750 mol}{1.500 L}=3.833 M](/tpl/images/0556/1625/31341.png)
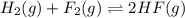
![K_c=\frac{[HF]^2}{[H_2][F_2]}](/tpl/images/0556/1625/a2854.png)
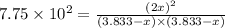
![[HF]=2x=2\times 3.576 M=7.152 M\approx 7.15 M](/tpl/images/0556/1625/f33c9.png)


