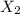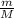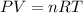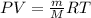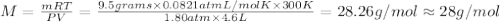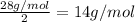To identify a diatomic gas (X2), a researcher carried out the following experiment: She weighed an empty 4.6-L bulb, then filled it with the gas at 1.80 atm and 27.0 ∘C and weighed it again. The difference in mass was 9.5 g . Identify the gas. Express your answer as a chemical formula. View Available Hint(s)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3


Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
To identify a diatomic gas (X2), a researcher carried out the following experiment: She weighed an e...
Questions


Mathematics, 24.11.2020 19:10





Arts, 24.11.2020 19:10


English, 24.11.2020 19:10


Arts, 24.11.2020 19:10


Mathematics, 24.11.2020 19:10






Mathematics, 24.11.2020 19:10

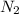 .
.