
Chemistry, 21.03.2020 03:15 gshreya2005
2.00 liters of hydrogen, originally at 25.0 °C and 750.0 mm of mercury, are heated until a volume of 20.0 liters and a pressure of 3.50 atmospheres is reached. What is the new temperature?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
2.00 liters of hydrogen, originally at 25.0 °C and 750.0 mm of mercury, are heated until a volume of...
Questions



Physics, 05.10.2019 17:30

Social Studies, 05.10.2019 17:30



History, 05.10.2019 17:30




Social Studies, 05.10.2019 17:30

Mathematics, 05.10.2019 17:30

Biology, 05.10.2019 17:30

Computers and Technology, 05.10.2019 17:30


Mathematics, 05.10.2019 17:30



Mathematics, 05.10.2019 17:30


 = initial pressure of gas = 750.0 mm Hg = 0.98 atm (760mmHg=1atm)
= initial pressure of gas = 750.0 mm Hg = 0.98 atm (760mmHg=1atm) = final pressure of gas = 3.50 atm
= final pressure of gas = 3.50 atm = initial volume of gas = 2.00 L
= initial volume of gas = 2.00 L = final volume of gas = 20.0 L
= final volume of gas = 20.0 L = initial temperature of gas =
= initial temperature of gas = 
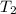 = final temperature of gas = ?
= final temperature of gas = ?



