
Chemistry, 21.03.2020 04:52 jackieanguiano3700
He following mechanism has been proposed for the gas-phase reaction of chloroform (CHCl3) and chlorine. Cl2 ⇌ 2Cl (fast, reversible) Cl + CHCl3 → HCl + CCl3 (slow) Cl + CCl3 → CCl4 (fast) What rate law does this mechanism predict? (Choose from the list below and enter your answers in alphabetical order, e. g. ABC ). A)k G) [CCl3]1/2 M) [HCl]2 B) [Cl] H) [HCl]1/2 N) [Cl2]2 C) [CHCl3] I) [Cl2]1/2 O) [Cl]2 D) [CCl3] J) [Cl]1/2 P) [CHCl3]2 E) [HCl] K) [CHCl3]1/2 F) [Cl2] L) [CCl3]2 Tries 0/99 Decide which of the following reactive intermediates could exist for the reaction above. Cl Cl2 CHCl3 CCl3 CCl4 Tries 0/99

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
He following mechanism has been proposed for the gas-phase reaction of chloroform (CHCl3) and chlori...
Questions

Arts, 16.07.2019 20:30



Biology, 16.07.2019 20:30





English, 16.07.2019 20:30

Biology, 16.07.2019 20:30

Biology, 16.07.2019 20:30




History, 16.07.2019 20:30

Mathematics, 16.07.2019 20:30

History, 16.07.2019 20:30






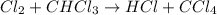
![\text{Rate}=K_2[Cl][CHCl_3]](/tpl/images/0557/3211/4079f.png) ......(1)
......(1)![K_1=\frac{[Cl]^2}{[Cl_2]}](/tpl/images/0557/3211/5c3f3.png)
![[Cl]=\sqrt{K_1[Cl_2]}](/tpl/images/0557/3211/af496.png)
![K_3=\frac{[CCl_4]}{[CHCl_3][Cl]}](/tpl/images/0557/3211/3404e.png)
![[Cl]=\frac{[CCl_4]}{K_3\times [CCl_3]}](/tpl/images/0557/3211/7b25a.png)
![\text{Rate}=K_2(\sqrt{K_1[Cl_2]}\times (\frac{[CCl_4]}{K_3[CCl_3]})[CHCl_3]\\\\\text{Rate}=k[Cl]^{1/2}[CCl_4][CCl_3]^{-1}[CHCl_3]](/tpl/images/0557/3211/b7240.png)


