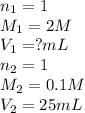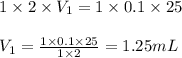Chemistry, 21.03.2020 05:05 Kittylover65
What volume (mL) of the partially neutralized stomach acid was neutralized by NaOH during the titration? (portion of 25.00 mL sample; this was the HCl remaining after the antacid tablet did it's job)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2


Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
What volume (mL) of the partially neutralized stomach acid was neutralized by NaOH during the titrat...
Questions

English, 06.04.2021 14:00



Mathematics, 06.04.2021 14:00


Mathematics, 06.04.2021 14:00



English, 06.04.2021 14:00


Mathematics, 06.04.2021 14:00

Social Studies, 06.04.2021 14:00

English, 06.04.2021 14:00


Business, 06.04.2021 14:00



Mathematics, 06.04.2021 14:00


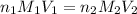
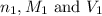 are the n-factor, molarity and volume of stomach acid which is HCl
are the n-factor, molarity and volume of stomach acid which is HCl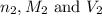 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.