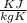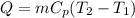Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
A sample of xenon gas at a pressure of 817 mm Hg and a temperature of 66 °C, occupies a volume of 13...
Questions



Mathematics, 18.05.2021 18:00



Mathematics, 18.05.2021 18:00

Business, 18.05.2021 18:00

Physics, 18.05.2021 18:00

Mathematics, 18.05.2021 18:00


Computers and Technology, 18.05.2021 18:00

History, 18.05.2021 18:00


Biology, 18.05.2021 18:00




Mathematics, 18.05.2021 18:00


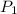 = 109 k pa = Final pressure
= 109 k pa = Final pressure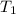 = 66°c = 339 K
= 66°c = 339 K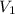 = 13.9 liter = 0.0139
= 13.9 liter = 0.0139 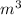
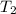 = 362 K
= 362 K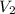 = 14.84 liter
= 14.84 liter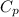 = 0.158
= 0.158