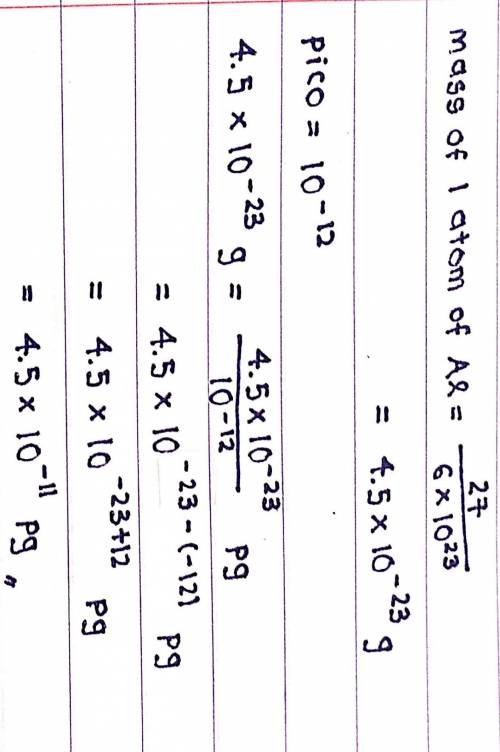
Chemistry, 21.03.2020 05:35 Frenchfries13
If 6.0 x 10^23 atoms of aluminum weigh 27 g, calculate the mass of 1 atom of aluminum in picograms.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
If 6.0 x 10^23 atoms of aluminum weigh 27 g, calculate the mass of 1 atom of aluminum in picograms....
Questions

Business, 01.02.2021 21:00


Mathematics, 01.02.2021 21:00



Biology, 01.02.2021 21:00

Mathematics, 01.02.2021 21:00


Mathematics, 01.02.2021 21:00

Mathematics, 01.02.2021 21:00







Social Studies, 01.02.2021 21:00

Mathematics, 01.02.2021 21:00


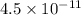 picograms
picograms