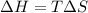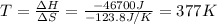Chemistry, 21.03.2020 05:35 milkshakegrande101
For a particular reaction, Δ H ∘ = − 46.7 kJ and Δ S ∘ = − 123.8 J/K. Assuming these values change very little with temperature, at what temperature does the reaction change from nonspontaneous to spontaneous?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
For a particular reaction, Δ H ∘ = − 46.7 kJ and Δ S ∘ = − 123.8 J/K. Assuming these values change v...
Questions



Mathematics, 20.04.2021 16:50

Mathematics, 20.04.2021 16:50

Mathematics, 20.04.2021 16:50





Chemistry, 20.04.2021 16:50



English, 20.04.2021 16:50

Social Studies, 20.04.2021 16:50



English, 20.04.2021 16:50

History, 20.04.2021 16:50


English, 20.04.2021 16:50

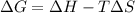
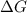 = Gibbs free energy
= Gibbs free energy 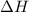 = enthalpy change = -46.7 kJ= -46700 J
= enthalpy change = -46.7 kJ= -46700 J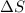 = entropy change = -123.8 J/K
= entropy change = -123.8 J/K