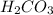Chemistry, 21.03.2020 08:24 BigGirlsTheBest
A mixture of a carboxylic acid and a phenol can often be separated by extracting with aqueous sodium bicarbonate and a suitable organic solvent. What difference in chemical properties of the carboxylic acid and the phenol make this separation possible?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
A mixture of a carboxylic acid and a phenol can often be separated by extracting with aqueous sodium...
Questions


Arts, 08.06.2021 19:30

Biology, 08.06.2021 19:30

Mathematics, 08.06.2021 19:30

Mathematics, 08.06.2021 19:30


Social Studies, 08.06.2021 19:30





Biology, 08.06.2021 19:30

Mathematics, 08.06.2021 19:30


Mathematics, 08.06.2021 19:30

Social Studies, 08.06.2021 19:30

Mathematics, 08.06.2021 19:30

Mathematics, 08.06.2021 19:30


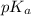 of carboxylic acid lies in the range of 1.92 - 4.76
of carboxylic acid lies in the range of 1.92 - 4.76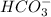 acts as a strong base because it is derived from weak conjugate acid
acts as a strong base because it is derived from weak conjugate acid