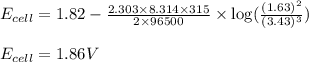A galvanic cell at a temperature of 42 degrees Celcius is powered by the following redox reaction:
3CU2+(aq) + 2Al(s) > 3Cu(s) + 2Al3+(aq)
Suppose the cell is prepared with 3.43 M Cu2+n one half-cell and 1.63 M Al3+in the other.
A) Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3


Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
A galvanic cell at a temperature of 42 degrees Celcius is powered by the following redox reaction:
Questions

Biology, 06.01.2020 23:31

Computers and Technology, 06.01.2020 23:31



Social Studies, 06.01.2020 23:31

Biology, 06.01.2020 23:31

Physics, 06.01.2020 23:31

Social Studies, 06.01.2020 23:31




Business, 06.01.2020 23:31








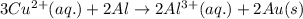
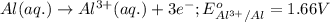 ( × 2)
( × 2)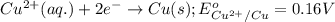 ( × 3)
( × 3)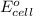 of the reaction, we use the equation:
of the reaction, we use the equation: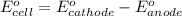
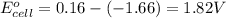
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Al^{3+}]^2}{[Cu^{2+}]^3}](/tpl/images/0557/5138/3aff8.png)
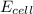 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![42^oC=[42+273]K=315K](/tpl/images/0557/5138/563a7.png)
![[Al^{3+}]=1.63M](/tpl/images/0557/5138/1e5e1.png)
![[Cu^{2+}]=3.43M](/tpl/images/0557/5138/455ea.png)