Chemistry, 21.03.2020 08:35 alexusjones6042
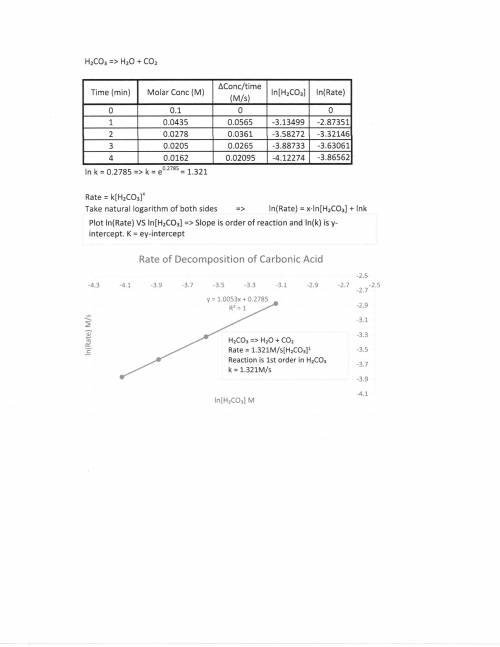
A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) →H2O(aq)+CO2(aq)
He fills a reaction vessel with H2CO3 and measures its concentration as the reaction proceeds:
time (minutes) H2CO3
0 0.100M
1.0 0.0435M
2.0 0.0278M
3.0 0.0205M
4.0 0.0162M
write the reate law for this reaction: k ?
calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbols.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) →H2O(aq)+CO2(aq)
Questions



Mathematics, 22.05.2020 08:03


Mathematics, 22.05.2020 08:03



Mathematics, 22.05.2020 08:03

Mathematics, 22.05.2020 08:03


Mathematics, 22.05.2020 08:03


Mathematics, 22.05.2020 08:03




Mathematics, 22.05.2020 08:03