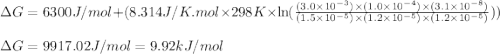Given the following: [G3P] = 1.5x10-5M; [BPG] = 3.0x10-3M ; [NAD+] = 1.2x10-5M; [NADH]=1.0x10-4 ; [HPO42-]= 1.2x10-5 M; pH = 7.5 ; DGo=6.3 kJ/mol
Glyceraldehyde3-phosphate + NAD+ + HPO42- ---> 1,3-Biphosphoglycerate + NADH + H+
Predict whether this reaction will be spontaneous.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1
You know the right answer?
Given the following: [G3P] = 1.5x10-5M; [BPG] = 3.0x10-3M ; [NAD+] = 1.2x10-5M; [NADH]=1.0x10-4 ; [H...
Questions










Mathematics, 26.02.2020 23:34

History, 26.02.2020 23:34





English, 26.02.2020 23:34




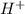 concentration, we use the equation:
concentration, we use the equation:![pH=-\log[H^+]](/tpl/images/0557/5671/cf945.png)
![7.5=-\log [H^+]](/tpl/images/0557/5671/95063.png)
![[H^+]=10^{-7.5)=3.1\times 10^{-8}M](/tpl/images/0557/5671/67b53.png)
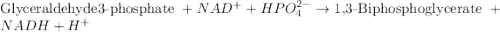
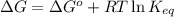
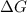 = free energy of the reaction
= free energy of the reaction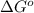 = standard Gibbs free energy = 6.3 kJ/mol = 6300 J/mol (Conversion factor: 1kJ = 1000J)
= standard Gibbs free energy = 6.3 kJ/mol = 6300 J/mol (Conversion factor: 1kJ = 1000J)![25^oC=[273+25]K=298K](/tpl/images/0557/5671/0e82f.png)
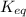 = Ratio of concentration of products and reactants =
= Ratio of concentration of products and reactants = ![\frac{[BPG][NaDH][H^+]}{[G_3P][NAD^+][HPO_4^{2-}]}](/tpl/images/0557/5671/dd6ba.png)
![[BPG]=3.0\times 10^{-3}M](/tpl/images/0557/5671/38e24.png)
![[NADH]=1.0\times 10^{-4}M](/tpl/images/0557/5671/94c0b.png)
![[H^+]=3.1\times 10^{-8}M](/tpl/images/0557/5671/2f9b6.png)
![[G_3P]=1.5\times 10^{-5}M](/tpl/images/0557/5671/aafff.png)
![[NAD^+]=1.2\times 10^{-5}M](/tpl/images/0557/5671/711eb.png)
![[HPO_4^{2-}]=1.2\times 10^{-5}M](/tpl/images/0557/5671/2ab39.png)