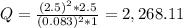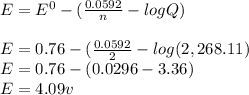Refer to the following standard reduction half-cell potentials at 25∘C:
VO2+(aq)+Ni2+(aq)2H+(aq)++2e−e−→ →Ni(s)VO2+(aq) +H2O(l)E∘=−0.23V E∘=0.99V
An electrochemical cell is based on these two half-reactions: Oxidation:Reduction:
Ni(s)VO2+(aq,0.083M)+2H+(aq,1.1M)+e −→→Ni2+(aq,2.5M)+2e−VO2+(aq,2.5M)+H 2O(l)
Calculate the cell potential under these nonstandard concentrations.
Express the cell potential to two decimal places and include the appropriate units.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1


Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

You know the right answer?
Refer to the following standard reduction half-cell potentials at 25∘C:
VO2+(aq)+Ni2+(a...
VO2+(aq)+Ni2+(a...
Questions

Mathematics, 08.03.2021 14:40






Mathematics, 08.03.2021 14:40

Mathematics, 08.03.2021 14:40


Mathematics, 08.03.2021 14:40

Mathematics, 08.03.2021 14:40

Mathematics, 08.03.2021 14:40

History, 08.03.2021 14:40


Law, 08.03.2021 14:40


Business, 08.03.2021 14:40

Biology, 08.03.2021 14:40


![E = E^{0} - [(\frac{0.0592}{n} · log Q)]](/tpl/images/0557/5710/84c6b.png)
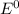 = Cell potential (standard conditions)
= Cell potential (standard conditions)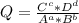
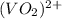 + e- -->
+ e- --> 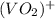 -0.23
-0.23 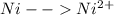 + 2 e- +0.99
+ 2 e- +0.99 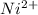 + 2e- 0.76 v
+ 2e- 0.76 v ![Q = \frac{[(VO_{2}+]^{2}* [Ni^{2+} }{[VO_{2+}]^{2} * Ni }]](/tpl/images/0557/5710/56e6b.png)
![[VO_{2} ^{2+}] = 2.5 M](/tpl/images/0557/5710/bdce1.png)
![[VO_{2}+] = 0.083 M](/tpl/images/0557/5710/9505a.png)
![[Ni^{2+}] = 2.5 M](/tpl/images/0557/5710/3a699.png)
![[Ni] = 1 M, it is a pure solid, so its activity in Q is unit (1). It is also applied for pure liquids.](/tpl/images/0557/5710/4494d.png)