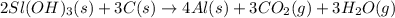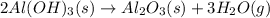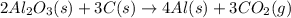Chemistry, 21.03.2020 10:56 jakhunter354
The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late 19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina and water: 2AlOH3(s)→Al2O3(s)+3H2O(g) In the second step, alumina and carbon react to form aluminum and carbon dioxide: 2Al2O3(s)+3C(s)→4Al(s)+3CO2(g) Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced.

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

You know the right answer?
The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult proc...
Questions




History, 22.09.2020 14:01


Spanish, 22.09.2020 14:01


English, 22.09.2020 14:01

Mathematics, 22.09.2020 14:01


English, 22.09.2020 14:01


Computers and Technology, 22.09.2020 14:01




English, 22.09.2020 14:01



Biology, 22.09.2020 14:01