
Chemistry, 21.03.2020 11:31 brenda2512
The following three equations represent reactions done in this lab exercise. Classify each reaction by their type. Note there may be more than one way to classify a reaction
1. Cu(s)+4HNO3(aq)⟶Cu(NO3)2(aq)+2NO2(g )+2H2O(l)
a. precipitation.
b. double displacement.
c. decomposition.
d. single displacement.
e. oxidation / reduction.
f. synthesis / combination.
2. Cu(NO3)2(aq)+2NaOH(aq)⟶Cu(OH)2(s)+2 NaNO3(aq)
a. synthesis / combination.
b. single displacement.
c. oxidation / reduction.
d. decomposition.
e. double displacement.
f. precipitation.
3. CuO(s)+2HCl(aq)⟶CuCl2(aq)+H2O(l)
a. precipitation.
b. double displacement.
c. synthesis / combination.
d. oxidation / reduction.
e. single displacement.
f. decomposition.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1



Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
The following three equations represent reactions done in this lab exercise. Classify each reaction...
Questions

Mathematics, 01.05.2021 14:00

Biology, 01.05.2021 14:00

Physics, 01.05.2021 14:00


Chemistry, 01.05.2021 14:00


Mathematics, 01.05.2021 14:00


Mathematics, 01.05.2021 14:00

English, 01.05.2021 14:00

Mathematics, 01.05.2021 14:00

Mathematics, 01.05.2021 14:00

Advanced Placement (AP), 01.05.2021 14:00

Mathematics, 01.05.2021 14:00

Geography, 01.05.2021 14:00

Mathematics, 01.05.2021 14:00


Mathematics, 01.05.2021 14:00

Mathematics, 01.05.2021 14:00

History, 01.05.2021 14:00

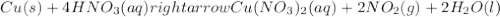 : oxidation reduction
: oxidation reduction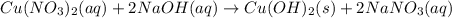 : precipitation
: precipitation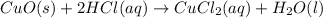 : Double displacement
: Double displacement

