
Chemistry, 21.03.2020 20:28 kragland4752
Use the electronegativities in the periodic table above to determine which bonding pair forms polar covalent bonds.
A) Lithium and Oxygen
B) Calcium and Chlorine
C) Nitrogen and Hydrogen
D) Hydrogen and Hydrogen
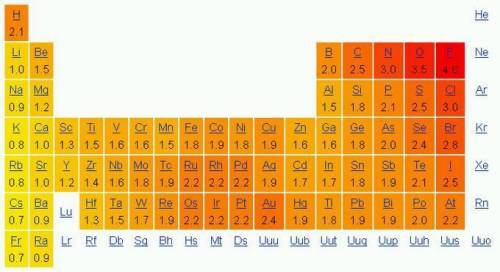

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1


Chemistry, 21.06.2019 23:30
Why do you suppose the structural polysaccharide cellulose does not contain branches? why do you suppose the structural polysaccharide cellulose does not contain branches? branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby decreasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby increasing the rigidity and strength of the microfibrils. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby increasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby decreasing the rigidity and strength of the microfibrils.
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
Use the electronegativities in the periodic table above to determine which bonding pair forms polar...
Questions

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Biology, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01



