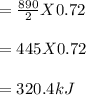Chemistry, 21.03.2020 22:19 annaebrown9737
How many kilojoules of heat are released when 0.72 mole of oxygen gas are used to combust methane?
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (ℓ) + 890kJ

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1


Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
How many kilojoules of heat are released when 0.72 mole of oxygen gas are used to combust methane?
Questions


English, 15.10.2019 06:30

Mathematics, 15.10.2019 06:30




Biology, 15.10.2019 06:30

English, 15.10.2019 06:30




Physics, 15.10.2019 06:30


History, 15.10.2019 06:30



History, 15.10.2019 06:30