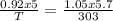A sample of argon has a volume of 5.0 dm^3 and the pressure is 0.92 atm.
If the final temperat...

Chemistry, 22.03.2020 02:39 dragon2998
A sample of argon has a volume of 5.0 dm^3 and the pressure is 0.92 atm.
If the final temperature is 30.° C, the final volume is 5.7 L, and the final
pressure is 800. mm Hg, what was the initial temperature of the argon?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
Questions

Arts, 22.12.2020 23:20

Mathematics, 22.12.2020 23:20




Chemistry, 22.12.2020 23:20

Mathematics, 22.12.2020 23:20

History, 22.12.2020 23:20

Mathematics, 22.12.2020 23:20




Arts, 22.12.2020 23:20


Mathematics, 22.12.2020 23:20

Computers and Technology, 22.12.2020 23:20

Mathematics, 22.12.2020 23:20



Mathematics, 22.12.2020 23:20

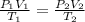
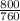 = 1.05atm
= 1.05atm