
Chemistry, 22.03.2020 05:00 Adolfosbaby
Calculate the maximum numbers of moles and grams of iodic acid (HIO3) that can form when 341 g of iodine trichloride reacts with 151.7 g of water:
ICl3 + H2O → ICl + HIO3 + HCl [unbalanced]

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
You know the right answer?
Calculate the maximum numbers of moles and grams of iodic acid (HIO3) that can form when 341 g of io...
Questions

Mathematics, 25.05.2020 03:57



Mathematics, 25.05.2020 03:57


Social Studies, 25.05.2020 03:57

Computers and Technology, 25.05.2020 03:57




Chemistry, 25.05.2020 03:57

Biology, 25.05.2020 03:57


Mathematics, 25.05.2020 03:57






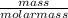
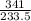 = 1.46moles
= 1.46moles 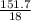 = 8.43moles
= 8.43moles 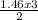 = 2.19 moles
= 2.19 moles 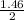 = 0.73moles
= 0.73moles 

