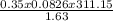Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

You know the right answer?
What volume of chlorine gas at 38oC and 1.63 atm is needed to react completely with 10.4 grams of so...
Questions


History, 05.10.2019 07:00


Business, 05.10.2019 07:00


Mathematics, 05.10.2019 07:00




Chemistry, 05.10.2019 07:00




English, 05.10.2019 07:00

Health, 05.10.2019 07:00

Spanish, 05.10.2019 07:00

History, 05.10.2019 07:00



Mathematics, 05.10.2019 07:00

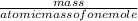
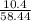
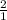 =
= 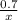
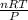 R=0.0826 L atm/Mole.K, T = 311.15, P= 1.63 atm, n= 0.35 moles
R=0.0826 L atm/Mole.K, T = 311.15, P= 1.63 atm, n= 0.35 moles