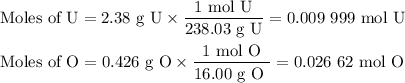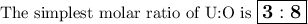2.38 grams of uranium is heated in a current of air. The resulting oxide
weighs 2.806 grams. W...

Chemistry, 23.03.2020 02:14 selenaK9514
2.38 grams of uranium is heated in a current of air. The resulting oxide
weighs 2.806 grams. When solving for empirical formula, the simplest
molar ratio of uranium is 1.
TRUE or
FALSE

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1


Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

You know the right answer?
Questions


English, 06.10.2021 07:40


Mathematics, 06.10.2021 07:40


Mathematics, 06.10.2021 07:40



Mathematics, 06.10.2021 07:40


Mathematics, 06.10.2021 07:40

Mathematics, 06.10.2021 07:40


Social Studies, 06.10.2021 07:40

Chemistry, 06.10.2021 07:40

History, 06.10.2021 07:40