
Chemistry, 23.03.2020 03:32 amandajbrewerdavis
Zinc reacts with iodine in a synthesis reaction. Using a balanced chemical equation for the reaction, determine the percent yield of a 129.0gram sample of zinc was used and 510.6 grams of product is recovered

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2

Chemistry, 23.06.2019 13:30
Which of these statements describes the size of an atom? a. an atom is larger than a sheet of aluminum foil. b. an atom is small but can be seen with just our eyes. c. an atom is the size of a plastic building block. d. an atom is tiny and cannot be seen without magnification.
Answers: 2

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1
You know the right answer?
Zinc reacts with iodine in a synthesis reaction. Using a balanced chemical equation for the reaction...
Questions

Advanced Placement (AP), 24.07.2019 09:50




English, 24.07.2019 09:50





Chemistry, 24.07.2019 09:50


Mathematics, 24.07.2019 09:50

History, 24.07.2019 09:50

Chemistry, 24.07.2019 09:50

History, 24.07.2019 09:50





Health, 24.07.2019 09:50

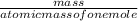
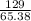
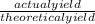 x100 equation 1
x100 equation 1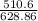 x 100
x 100

