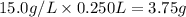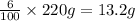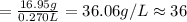Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2


Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
250 mL of a salt solution with a concentration of 15.0 g/L is mixed with 220 mL of a salt solution c...
Questions

Health, 01.12.2020 19:30



Mathematics, 01.12.2020 19:30

Spanish, 01.12.2020 19:30

Chemistry, 01.12.2020 19:30


Mathematics, 01.12.2020 19:30



Mathematics, 01.12.2020 19:30

Physics, 01.12.2020 19:30

History, 01.12.2020 19:30





Chemistry, 01.12.2020 19:30